Cell Culture Reagents
Market-leading Cell Culture Reagents from BBI
We manufacture every transferrin supplement under ISO13485 certification at our UK facility.
Human transferrin sits at the heart of modern serum-free media. BBI has produced Holo and Apo transferrin in tightly controlled 5 kg batches. Plasma from FDA-licensed US donors is heat-pasteurized to WHO guidelines, 0.2 µm-filtered, lyophilised and exhaustively characterised for iron content, endotoxin and purity. The result is a safe, consistent iron-carrier that accelerates cell growth and scales smoothly from discovery flasks to commercial bioreactors.
Transferrin
Iron-saturated Holo and iron-poor Apo transferrin, produced in 5 kg ISO 13485 lots from FDA-screened plasma, deliver safe, consistent iron for high-density serum-free cultures.
Brochures
Brochure
Products & Services
Inside this catalog you’ll find one of the industry’s broadest collections of enzymes, antibodies, antigens, and more – meticulously developed to power world-class diagnostics and keep your pipeline moving.
Download
Brochure
Reagents Range
Discover our collection of reagents covering a comprehensive range of biomarkers and applications for the IVD market, life sciences, cell culture and beyond.
Download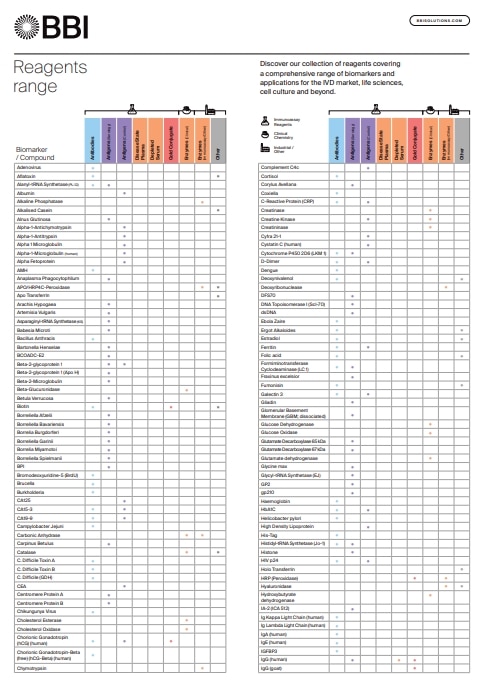



Frequently asked questions
Cell Cultures
What are the different forms of Transferrin available?
The standard Transferrin products supplied by BBI are lyophilised products, material is available with different levels of iron saturation. Circulating Transferrin is partially Iron saturated however for cell culture use BBI adjusts the iron content to provide the following options:
Holo-transferrin (product code T101-5) is adjusted to give near 100% saturation (1200-1700 µg/gm). Apo-transferrin (product code T100-5) is depleted to give near zero iron bound (<50µg/gm). Customised Transferrin formulations are available on request.
What controls are in place for the collection of the blood used as the raw material?
Human plasma used for the manufacture of the BBI product human transferrin is collected at blood centers in the United States of America which are licensed by the United States Food and Drug Administration (FDA). The material is manufactured from a large pool of plasma donated by multiple donors, although the donor’s information is not made available, all donations are traceable back to the donor. All plasma donations are screened for infectious diseases by FDA approved methods at the donor level. Donations are ethically sourced and informed consent forms are signed by each donor prior to donation, the forms are kept on file by the approved collection facility. Further details on the collection process are available on specific information sheets or can be requested from technicalsupport@bbisolutions.com.
What regulatory system covers the Transferrin production? Are the transferrin products GMP/ clinical grade products and can it be used for therapeutic products?
BBI prepares Transferrin under our ISO13485 quality system however the transferrin products are prepared in open laboratories and the material is not GMP/clinical grade. Transferrin is intended for research and cell culture use only and is not suitable for use in pharmaceuticals.
How do I store the supplied material?
Transferrin products should be stored at 2-8°C. After the Transferrin has been removed from the sealed packaging the material should be used immediately, it should not be stored for re-use.
How is the shelf-life of the products determined?
A shelf-life of a minimum of 5 years from manufacture date is applied. The shelf life is based on immunological potency.
How can I order/sample the products?
These requests can be sent directly to your designated account manager who will be happy to advise of current stock availability and advise on appropriate samples. If you do not have an account manager, contact customer services.
How can I get further information about the product?
If any further technical information is required, this can be requested by emailing technicalsupport@bbisolutions.com or contacting customer services.
How is the material purified?
The specific methodologies are proprietary to BBI however a general purification methodology can be provided after execution of a Non-disclosure agreement.
What is Transferrin and how is it used?
Human transferrin is a major constituent of blood plasma. It is an iron binding glycoprotein that serves as the transport protein for iron delivery in the body. Each molecule of transferrin specifically binds two Fe3+ molecules through a bicarbonate mediated site-specific binding. Transferrin is required by many mammalian cells during growth to regulate iron uptake and it has been shown that when added to cell culture media it promotes cell proliferation. In culture media, transferrin has a secondary role to bind endogenous metal ions which may cause cell toxicity.
What is the source material for the Transferrin?
BBI derives it’s purified Transferrin products from pooled normal human plasma from predominantly US based paid blood donations.
Request a sample
IN FOCUS
ISO Certifications
All our cell culture media components are manufactured at facilities certified to internationally recognised ISO13485 quality management standards – so your diagnostics start with a proven quality backbone. Our ISO27001 certification ensures your data is safeguarded, and we’re now charting a path to ISO 14001 and ISO 45001 to fold sustainability and safety into every batch.
ISO Certifications
ADDING VALUE
Performance
Backed by 70 years of reagent expertise, BBI products are engineered for consistent, assay-grade performance - batch after batch.
Scalability
From pilot-scale to full scale industrial runs, our bioprocesses and global logistics flex with your forecasts, ensuring on-spec supply worldwide.
Compliance
ISO 13485 certification plus FDA registration and full documentation make every lot audit-ready - speeding global approvals and market entry.
Security
Global primary manufacturing and secure supply chains ensure security of supply, so your production timeline stays protected.
Recently Viewed
GET IN TOUCH
Still need help? Talk to our team
Our customer service team is on hand to help with any questions you have or support you need.
And if the issue is technical, they’ll bring in our world-leading expert scientists to troubleshoot side-by-side with you and keep your project moving.
Contact us